Berberine treats high blood sugar and bad lipids
A systemic review and meta-analysis finds antidiabetic benefits …
It is surprising to note that many physicians fear the responsibility of knowing new clinical data|
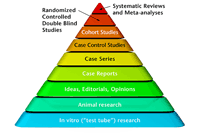
Figure 1. The evidence-base pyramid(click on thumbnail for full sized image) |
In a course titled “What Is Evidence-Based Medicine?” offered at the State University of New York Downstate Medical Center (see sidebar, “SUNY’s Nobel sage: NO is beautiful”) a number of outstanding points are made.1Bear in mind that this course was designed for medical students, who are expected to be reactionary (reacting to pain, suffering, and disease), rather than proactionary (concerned with prevention).
Clinicians fear judgment nullification by Medline
Evidence-based medicine may be thought of as the integration of individual clinical expertise with the best available external clinical evidence from systemic research. Yet many clinicians fear that their judgment is nullified by practicing medicine via Medline* searches. Their anxieties fly in the face of the fact that clinical expertise still plays a large part in any decision made on treatment, diagnosis, screening, etc. It’s just that many clinicians feel helpless when imposed upon to learn a new skill, and constantly be integrating new data (“I thought I was through with medical school!”).
*Medline is the largest component of PubMed (http://pubmed.gov /), the freely accessible online database of biomedical journal citations and abstracts created by the U.S. National Library of Medicine (NLM). Approximately 5,400 journals published in the United States and more than 80 other countries have been selected and are currently indexed for Medline. A distinctive feature of Medline is that the records are indexed with NLM’s controlled vocabulary, the Medical Subject Headings.
As with clinicians, so with patients … fear
On the other hand, many patients also express fear that clinicians are just making guesses and hoping for the best. However, this fear may be dampened by reliance on strictly codified criteria of what constitutes good evidence and how to find it (certainly not through professional networks or through anything other than the primary literature). In other words, if clinical judgment is sharpened by good evidence, and clinicians develop the ability to determine what constitutes good evidence, then judgment and findings are integrated and complementary. They thus represent valuable medical assets. According to the Evidence-Based Medicine course, “If you are to remain a good doctor, or become a better one, you need to stay on top of new developments as they occur. Evidence-based medicine provides you with the tools you need to find important new medical research quickly and easily, and to work out its implications for your practice.”
Berberine clinical studies and the standards of research
Turning to a new Chinese systemic review and meta-analysis about the use of berberine for type 2 diabetes (T2D), this study seems to fit the top category of the evidential pyramid shown above [see Fig. 1].2 Or does it? As we shall see, there are limitations to the reliability of the evidence-based pyramid (which is based on drug standards), when it comes to nutritional supplements.
The prevalence of T2D has continued to increase the world over. In fact, the International Diabetes Federation computes the number of diabetics in 2011 to have reached an astounding 366 million, with 4.6 million deaths attributable to the disease in 2011.3 While diet and exercise may help prevent the onslaught of T2D, the use of oral hypoglycemic agents and potentially subcutaneous insulin injections are considered to be valuable, and the first conventional approach for treating T2D. As evidence from multicenter trials has demonstrated, drugs are able to lower blood glucose and to reduce the risk of developing diabetic complications. Yet they have long-term side effects, including a high incidence of gastrointestinal side effects with the use of metformin, and an increased risk of cardiovascular disease with the use of glitazones. This information may not be sufficiently weighted when examining the pyramid of evidence.
Setting the stage for complementary therapies
Consequently, many diabetic patients who take diabetic drugs are also taking complementary and alternative medicine therapies, or are only using herbal therapies. This is particularly true in China, where T2D is referred to as “Xiao Ke” disease (translates as emaciation and thirst), which is frequently a consequence of high-glycemic diets and sedentary lifestyles. There is a long and continuous history of herbal medication use to treat diabetes in China where considerable research presents a strong case that Rhizoma coptidis (which contains berberine as its major constituent) can help those with T2D by reducing glucose activities and decreasing bad lipids.
Berberine is an alkaloid† isolated from R. coptidis, an herb that has been widely used to treat gastrointestinal infections (such as bacterial diarrhea). The content of berberine in R. coptidis ranges from about 5% to 8%. Nor is the scientific research on berberinenew. Its blood-sugar lowering effect (hypoglycemic) was first reported in 1988, when it was used to treat diarrhea in diabetic patients. Indeed, over the years, the anti-hyperglycemic effect of berberine has been recognized by many Chinese physicians, as demonstrated by reports of clinical trials in medical journals.
†Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. Humans have used alkaloid-containing plants since ancient times for therapeutic purposes. For example, in The Odyssey of Homer, Helen was given Nepenthe, a opium-containing herb by the Egyptian Queen, to induce oblivion. Further along, Odysseus is given Moly, now thought to be Galanthus nivalus (a galantamine-containing herb), to defend his memory against poisoning by henbane (an atropine-containing plant). All are alkaloids.
Evidence-based medicine may be
thought of as the integration of
individual clinical expertise with the
best available external clinical
evidence from systemic research.
Mechanisms of berberine
At the same time, numerous other studies have concentrated on discovering the molecular mechanisms underlying the effects of berberine. The key findings support several antidiabetic actions of berberine. These include the increased secretion of insulin, improved insulin resistance, and reduced bad lipids (dyslipidemia). The hypoglycemic effect of berberine is also partially mediated by an anti-inflammatory mechanism. This finding adds new evidence indicating that T2D is a low-grade inflammatory disease.
The issues of qualifying berberine for T2D
While berberine is effective on improving high blood-sugar levels (hyperglycemia), there exist a number of issues. To date, there are few multicenter clinical trials to confirm its hypoglycemic action in a larger number of patients. And this may never happen. Drugs are much more profitable than herbs and thus the money is available to finance large-scale studies. The profit stems from the fact that drugs are patentable and herbs are not.
There are limitations to the reliability
of the evidence-based pyramid (which
is based on drug standards) when it
comes to nutritional supplements.
As well, scientific evidence showing that berberine is as effective as other conventional treatments (drugs) in treating T2D remains to be further validated. This has happened, but not on a large-scale basis, again because of the costs. In terms of safety concerns, there is no absolute certainty about the safety of a long-term berberine intake for the treatment of diabetes. This concern has been raised since the initial application of berberine as an anti-diarrhea agent. More to the point, but far from convincing, there have been reports of berberine-induced hemolysis in chronic hematological diseases. But we do know that we can be certain that the long-term use of diabetic drugs is not safe (see examples of metformin and glitazones above).
The scope of the Chinese review and meta-analysis
In the Chinese paper, the researchers identified seventeen randomized-control trials(RCTs) involving berberine and T2D. Three trials were excluded due to a tremendous difference in the number of patients found between two-armed parallel groups in two studies, and for an uncertain description of using the oral hypoglycemics in a control group in the third excluded study. Fourteen RCTs met the inclusion criteria; the number of subjects in these trials totaled 1068 patients.
The included studies (all Chinese) were published as full text between 2007 and 2011. Three studies were published in English and the remaining eleven studies were published in Chinese. Thirteen of the fourteen trials were performed as single center trials, while one study was a multicenter trial. Nine trials adopted a two-armed parallel group design, while five trials adopted a three- or four-armed group design. Two studies designed three parallel groups, which were glipizide (an oral rapid- and short-acting anti-diabetic drug from the sulfonylurea class), berberine, and the combination of both test materials. Three parallel groups were also found in another study, which were metformin, berberine, and the combination of both. Three parallel groups were included in the design of yet another, which were lifestyle modification plus placebo, lifestyle modification with aspirin, and lifestyle modification with berberine. In yet another, three parallel groups were included, which were berberine, metformin, and rosiglitazone. Then there were four parallel groups in one more study. The interventions were lifestyle modification alone, lifestyle modification with metformin, lifestyle modification with berberine, and lifestyle modification with berberine accompanied by a Chinese herbal formulation.
Berberine and lifestyle
Four studies randomized participants to receive berberine with a co-intervention of lifestyle modification versus a control of lifestyle modification alone or plus a placebo. Seven trials compared berberine with one kind of oral hypoglycemic drugs (metformin, glipizide, or rosiglitazone). Four trials compared a co-intervention of berberine and one type of oral hypoglycemics (metformin, glipizide) with a control of the same hypoglycemics. Two trials compared a co-intervention of berberine and two types of oral hypoglycemics (metformin, glipizide, or glimepiride) with a control of the same hypoglycemics.
Drugs are much more profitable than
herbs and thus the money is available
to finance large-scale studies. The
profit stems from the fact that drugs
are patentable and herbs are not.
Common dosage of berberine
The dose of berberine used in the included trials was different, but intake of berberinewas generally in a range between 0.5 g and 1.5 g per day. The total daily berberineintake was divided into two or three doses. However, one study varied the dose to body weight (20 mg per kilogram for each participant) and another employed three different doses of berberine based on fasting plasma glucose (FPG) levels. The dose was stable and remained unchanged during the period of study in twelve trials, while the remaining two trials reduced the dose of berberine during the period of study when the gastrointestinal discomfort occurred. The duration of interventions in the included trials was also different, ranging from eight weeks (three trials) to twelve or fourteen weeks (ten trials) and twenty-four weeks (one trial).
Intake of berberine was generally in a
range between 0.5 g and 1.5 g per day.
The effects of berberine on diabetes
All the included trials were performed to evaluate hypoglycemic and/or anti-dyslipidemic effects of berberine. The outcomes reported were what are called surrogate parameters. These included FPG, glycosylated hemoglobin levels A1c (HbA1c), fasting insulin levels (FINS), and plasma lipids. Miner diverse effects were reported in eleven trials. All the reported outcomes were measured at the end of the intervention. Six trials showed the primary and the secondary outcomes completely. The rest of the trials only presented a part of the outcomes. Thirteen trials performed treated-per-protocol analysis, and one performed intention-to-treat analysis. A statistician combined the outcomes of the study with metformin and rosiglitazone groups. The study that used berberine and the Chinese herbal formulation was not included because it did not meeting the inclusion criteria. For the same reason, the outcomes of the aspirin group were not included. Co-intervention of anti-dyslipidemia agents was used in another trial and the outcomes of plasma lipids were also not included. This was because the plasma lipids data was similar to total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C).
|
SUNY’s Nobel sage: NO is beautiful At the State University of New York Downstate Medical Center, Dr. Robert Furchgott, as a professor of pharmacology from 1956 to 2009, did the work that led to the Nobel Prize in Physiology or Medicine in 1998, which he shared with professors Louis Ignarro and Ferid Murad. The prize was awarded principally for Furchgott’s discovery of a substance in endothelial cells that relaxes blood vessels, calling it endothelium-derived relaxing factor (EDRF). By 1986, he had determined EDRF’s nature and mechanism of action, and identified that EDRF was in fact nitric oxide (NO), an important compound in many aspects of cardiovascular physiology. This research was important in explaining the action of Viagra, treatment of blue babies, and the role of NO in cardiovascular function, among other medical and health-related issues. In his Nobel banquet speech, Dr. Furchgott sagely noted that Alfred Nobel had been diagnosed with angina in the last ten years of his life and been prescribed nitroglycerin, which achieves its benefits by the release of NO. The fortune of Nobel had been largely based on the use of nitroglycerin as the principal component of dynamite. Nobel was its discoverer. During most of his life, Alfred Nobel suffered from poor health. He complained of indigestion, headaches and occasional spells of depression. As a young man, he spent several weeks at health resorts. His first stay at a spa was at Franzensbad in Bohemia in 1854. The inactivity at the health resorts made him restless and bored and he could not have been impressed by the medical treatment offered at the spas. It consisted of baths, resting, and drinking well water. So he left all too soon, without receiving the full benefits of the high-content lithium water [see article on page 4], which could have significantly reduced the stress from which he continually suffered, and help turn his health around. |
Poor quality indicated by suggested bias
Most of the included trials in this meta-analysis were of poor quality, as indicated by unclear random sequence generation, inadequate allocation concealment, inadequate blinding, and failure to describe withdrawal or dropouts, suggesting high risk of bias. Only one trial performed a randomized, double-blind, placebo-controlled trial in four centers, where expenses were not an issue.
The current review indicates that
berberine with lifestyle modification
was more effective in terms of
lowering FBG compared with lifestyle
intervention alone, or plus placebo.
Randomization was also performed centrally and was concealed and stratified in blocks of four. Other potential sources of bias in the included studies included variation in the dose, duration, and type of the interventions. In addition, the methodological heterogeneity may arise through the diversity of results, which was attributed to different laboratory methods for the parameter determinations.
Berberine with a co-intervention of lifestyle modification
Four trials (involving 271 patients) evaluated the therapeutic effect of berberine with a co-intervention of lifestyle modification versus a control of lifestyle modification in the presence or not of placebo as the control. The number of trial participants ranged from 20 to 58 participants, with the trial duration lasting 12 weeks. The statistical heterogeneity between the studies was significant among the results of FINS, TC, and LDL-C. Pooled results showed a significant difference between the berberine-treated group and the control group. Berberine with a co-intervention of lifestyle modification was better than lifestyle modification alone or plus placebo in terms of improving FPG levels, and HbA1c. Meanwhile, plasma levels of triglycerides (TG) and LDL-C were significantly decreased and high-density lipoprotein cholesterol (HDL-C) levels were increased. To a smaller extent, plasma levels of TC and FINS were decreased in berberine with a co-intervention of lifestyle modification group.
Berberine versus oral hypoglycemics
Seven trials (involving 448 patients) compared berberine with oral hypoglycemics. The number of trial participants ranged from 15 to 51 with trial duration ranging from 8 to 13 weeks. In the pool of results on metabolic measures there was remarkable statistical heterogeneity among the comparisons, particularly postprandial plasma glucose (PPG) levels, FINS, TG, and HDL-C. No meta-analysis was conducted due to considerable statistical heterogeneity. As a consequence, the researchers described the outcomes of the trials included separately.
Three trials did not report the difference in PPG levels between the berberine and control groups. One trial showed that berberine was worse than the control in terms of reducing PPG levels, whereas one trial showed that berberine appeared to be better than the control group with regard to reducing PPG levels. Two trials did not report the difference in FINS levels between the berberine and control groups. One trial showed that berberine was worse than the control in terms of reducing FINS levels. In contrast, one trial showed that berberine has a better efficacy than the control in terms of reducing FINS levels.
Two trials did not compare TG levels in the berberine group with those in the control group. Two trials reported that berberine was better than the control in terms of reducing TG levels. However, one trial showed that there was no significant difference in TG outcomes between the berberine and control groups. No data were available for differences in HDL-C levels between the berberine and control groups in two trials. Two other trials reported that there was no significant difference in HDL-C outcomes between the berberine and control groups.
Also, there was no significant difference between berberine and oral hypoglycemics in terms of reducing FPG and HbA1c. Nonetheless, compared with those taking oral hypoglycemics, patients who took berberine showed significantly better results of TC.
Berberine combined with oral hypoglycemics versus the same oral hypoglycemics alone
Six trials (involving 396 patients) compared a co-intervention of berberine and oral hypoglycemics with the same oral hypoglycemics alone. The number of trial participants ranged from 17 to 51 with the trial duration ranged from 8 to 24 weeks. The statistical heterogeneity between the studies was significant in the results of PPG, HbA1c, FINS, TG, and LDL-C. There was a significant improvement in FPG, PPG, HbA1c, and FINS in patients who took berberine and oral hypoglycemics. In two of these trials, combination of berberine and oral hypoglycemics did not significantly improve TG, LDLC and HDL-C levels compared with the control group. Plasma TC levels were moderately reduced after the addition of berberine.
Low Adverse effects from berberine
Eleven of fourteen trials reported outcomes for adverse effects, whereas the remaining three reported no adverse effects during the berberine treatment. Three of these reported the incidence of abdominal discomfort—including nausea, abdominal distension, and diarrhea—but did not report the group in which abdominal discomfort occurred. Five trials mentioned in detail that the adverse effects occurred in the berberine intervention group.
Curiously, shifting berberine consumption to after a meal relieved the abdominal discomfort in one trial. Other researchers reported that only a few patients developed a mild diarrhea caused by the intake of berberine. In another trial, the constipation in three participants in the berberine group was relieved without dose reduction, and two patients with mild constipation reduced the berberine dose to achieve relief. No severe hypoglycemia was observed in any of the included trials, and perhaps of most importance, there was no significant difference between the berberine and the control groups regarding the incidence of adverse effects. No serious adverse events were observed.
What was learned
Although many clinical trials regarding the antidiabetic effect of berberine have been conducted, a systemic review was firstly reported in January 2012.4 The author of this analysis also published the same abstract in the supplementary issue of Diabetes. Ten RCTs involving 647 Chinese patients with T2D were included in their review, but no meta-analysis was performed due to significant statistical heterogeneity.
Thus, the authors only reported the outcomes of each trial. Compared with this previous review, the Chinese researchers of the current review and meta-analysis added another four studies. Following this, they set three subgroups in order to minimize the heterogeneity, which resulted in combing the data successfully and enabled the performing of a meta-analysis.
These outcomes suggest that
berberine has a potential
hypoglycemic effect, which seems to
be as effective as the conventional
oral hypoglycemics.
Thus, the current review indicates that berberine with lifestyle modification was more effective in terms of lowering FBG compared with lifestyle intervention alone or plus placebo. Also, berberine was found to have a much better effect on reducing PPG and HbA1c levels. With regard to lowering glucose and HbA1c, berberine appeared to improve blood glucose control in terms of normalization or an obvious reduction. The similar glycemic control was observed when berberine was compared with the conventional antidiabetic therapy.
Furthermore, berberine showed additional hypoglycemic effect when combined with the antidiabetic agents. These outcomes suggest that berberine has a potential hypoglycemic effect, which seems to be as effective as the conventional oral hypoglycemics.
Some of the plasma lipid profiles in diabetic patients were improved by the berberineintake during a two- or three-month period. When berberine and lifestyle modification were compared with lifestyle modification, alone or plus placebo, plasma levels of TG, TC, and LDL-C were decreased and HDL-C was increased, indicating an additional effect on dyslipidemia. However, compared with oral hypoglycemics, those taking berberine just showed better results for TC, and LDL-C without any effect on HDL-C. The result of plasma TG level was unclear. Generally, berberine appeared to have an additional cholesterol-lowering effect in treating diabetes, which was also found in the patients with hyperlipidemia.
This systematic review also has several limitations. First, all trials included were conducted among Chinese participants in the mainland of China, and thus there was a high risk of selection bias, meaning that the results were not valid and applicable to other ethnic origins. Second, most of the studies were of poor quality. Only one study was double-blinded and performed adequate allocation concealment. Two studies did not use blinding but performed unclear allocation concealment. The remaining eleven studies did not use blinding or allocation concealment. Thus, potential bias in selection of patients, administration of treatment, and assessment of outcomes could lead to overestimation of the therapeutic efficacy of berberine. Third, the limited number (from 4 to 7) of the trials included in each subgroup constrained the positive evidence of berberine for diabetes.
During data extraction, the Chinese researchers also found that only two or three studies provided the available data, especially for the outcomes related to plasma lipids. Quantitative subgroup analyses should not be performed when lacking insufficient data. The latter was also the reason why they were not able to draw a solid conclusion about the efficacy of berberine on dyslipidemia.
Lastly, the heterogeneity between the trials included in each subgroup was also significant. It arose through the differences in the type of control method, dose of treatment, and duration of intervention of the included studies. The variation of participant age, gender, and blood glucose level at baseline may have additionally contributed to heterogeneous results. Therefore, all of the outcomes should be carefully interpreted based on substantial methodological and clinical diversity.
In the end …
Based on the existing evidence reviewed, berberine has beneficial effects on blood glucose control in the treatment of T2D patients and exhibits efficacy comparable with that of conventional oral hypoglycemics. The anti-dyslipidemia effect of berberineneeds to be further confirmed. Additionally, berberine has no serious adverse effects except for a mild to moderate gastrointestinal discomfort. Due to the lack of high quality clinical trials, the efficacy of berberine at treating diabetes remains to be validated. This is particularly true for the effect of berberine on improving dyslipidemia in T2D.
Based on the existing evidence
reviewed, berberine has beneficial
effects on blood glucose control in
the treatment of T2D patients and
exhibits efficacy comparable with that
of conventional oral hypoglycemics.
That said, what about the use of berberine for prevention, rather than recovery? How likely is it that research to determine this will be done? The bias of the evidence-based pyramid, as indicated in the opening paragraph of this article hinges on its reactionary (reacting to pain, suffering, and disease), rather than proactionary (concerned with prevention) stance.
References
- SUNY Downstate Medical Center Web site: What Is Evidence-Based Medicine? Available at http://library.downstate.edu/EBM2/intro.htm /. Acccessed November 12, 2012.
- Dong H, Wang N, Zhao L, Lu F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evid Based Complement Alternat Med 2012;2012:591654. doi: 10.1155/2012/591654. Epub 2012 Oct 15.
- International Diabetes Federation Web site: IDF Diabetes Atlas.http://www.idf.org/diabetesatlas/5e/the-global-burden . Accessed: November 14, 2012.
- Na Q, Zhao TY, He M, TianC. Effectiveness and safety of berberine in the treatment of type 2 diabetes: a systematic review. Chin J Evid-Based Med2012;12(1):81–91.